
SI base unit
Background Information
This wikipedia selection has been chosen by volunteers helping SOS Children from Wikipedia for this Wikipedia Selection for schools. All children available for child sponsorship from SOS Children are looked after in a family home by the charity. Read more...
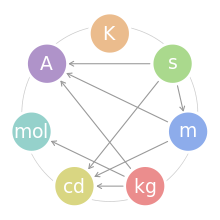
The International System of Units (SI) defines seven units of measure as a basic set from which all other SI units are derived. These SI base units and their physical quantities are:
- metre for length (US English: meter)
- kilogram for mass (note: not the gram)
- second for time
- ampere for electric current
- kelvin for temperature
- candela for luminous intensity
- mole for the amount of substance.
The SI base quantities form a set of mutually independent dimensions as required by dimensional analysis commonly employed in science and technology. However, in a given realization of these units they may well be interdependent, i.e. defined in terms of each other.
The names of SI units are written in lowercase characters (the 'degree Celsius' meets this rule, as 'degree' is the unit, and 'Celsius' is a modifier). The symbols of units are written in lowercase (e.g. metre has the symbol m), except that symbols for units named after persons are written with an initial capital letter (e.g., the hertz has the uppercase symbol Hz).
Many other units, such as the litre (US English: liter), are formally not part of the SI, but are accepted for use with SI.
The seven SI base units
| Name | Symbol | Measure | Current (2005) formal definition | Historical origin / justification |
|---|---|---|---|---|
| metre | m | length | "The metre is the length of the path travelled by light in vacuum during a time interval of 1 ⁄ 299,792,458 of a second." 17th CGPM (1983, Resolution 1, CR, 97) |
1 ⁄ 10,000,000 of the distance from the Earth's equator to the North Pole measured on the circumference through Paris. |
| kilogram | kg | mass | "The kilogram is the unit of mass; it is equal to the mass of the international prototype of the kilogram." 3rd CGPM (1901, CR, 70) |
The mass of one litre of water. A litre is one thousandth of a cubic metre. |
| second | s | time | "The second is the duration of 9,192,631,770 periods of the radiation corresponding to the transition between the two hyperfine levels of the ground state of the caesium 133 atom." 13th CGPM (1967/68, Resolution 1; CR, 103) "This definition refers to a caesium atom at rest at a temperature of 0 K." (Added by CIPM in 1997) |
The day is divided in 24 hours, each hour divided in 60 minutes, each minute divided in 60 seconds. A second is 1 ⁄ (24 × 60 × 60) of the day |
| ampere | A | electric current | "The ampere is that constant current which, if maintained in two straight parallel conductors of infinite length, of negligible circular cross-section, and placed 1 metre apart in vacuum, would produce between these conductors a force equal to 2 × 10−7 newton per metre of length." 9th CGPM (1948) |
The original "International Ampere" was defined electrochemically as the current required to deposit 1.118 milligrams of silver per second from a solution of silver nitrate. Compared to the SI ampere, the difference is 0.015%. |
| kelvin | K | thermodynamic temperature | "The kelvin, unit of thermodynamic temperature, is the fraction 1 ⁄ 273.16 of the thermodynamic temperature of the triple point of water." 13th CGPM (1967/68, Resolution 4; CR, 104) "This definition refers to water having the isotopic composition defined exactly by the following amount of substance ratios: 0.000 155 76 mole of 2H per mole of 1H, 0.000 379 9 mole of 17O per mole of 16O, and 0.002 005 2 mole of 18O per mole of 16O." (Added by CIPM in 2005) |
The Celsius scale: the Kelvin scale uses the degree Celsius for its unit increment, but is a thermodynamic scale (0 K is absolute zero). |
| mole | mol | amount of substance | "1. The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon 12; its symbol is 'mol.' 2. When the mole is used, the elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles." |
Atomic weight or molecular weight divided by the molar mass constant, 1 g/mol. |
| candela | cd | luminous intensity | "The candela is the luminous intensity, in a given direction, of a source that emits monochromatic radiation of frequency 540×1012 hertz and that has a radiant intensity in that direction of 1/683 watt per steradian." 16th CGPM (1979, Resolution 3; CR, 100) |
The candlepower, which is based on the light emitted from a burning candle of standard properties. |
Proposed redefinitions
There have been several modifications to the definitions of the base units, and additions of base units, since the Metre Convention in 1875. Since the redefinition of the metre in 1960, the kilogram is the only unit which is directly defined in terms of a physical artifact rather than a property of nature. However, the mole, the ampere and the candela are also linked through their definitions to the mass of this platinum–iridium cylinder stored in a vault near Paris. It has long been an objective of metrology to find a way to define the kilogram in terms of a fundamental constant, in the same way that the metre is now defined in terms of the speed of light.
The 21st General Conference on Weights and Measures (CGPM, 1999) placed these efforts on an official footing, and recommended "that national laboratories continue their efforts to refine experiments that link the unit of mass to fundamental or atomic constants with a view to a future redefinition of the kilogram." Two main possibilities have attracted attention: the Planck constant and the Avogadro constant.
In 2005, the International Committee for Weights and Measures (CIPM) approved the preparation of new definitions for the kilogram, the ampere, and the kelvin and it noted the possibility of a new definition for the mole based on the Avogadro constant. The 23rd CGPM (2007) decided to postpone any formal change until the next General Conference in 2011.
In a note to the CIPM in October 2009, Ian Mills, the President of the CIPM Consultative Committee - Units (CCU) cataloged the uncertainties of the fundamental constants of physics according to the current definitions and their values under the proposed new definition. He urged the CIPM to accept the proposed changes in the definition of the kilogram, ampere, kelvin and mole so that they are referenced to the values of the fundamental constants, namely Planck's constant (h), the electron charge (e), Boltzmann's constant (k), and Avogadro's constant (NA).
