Antimony
About this schools Wikipedia selection
SOS Children, an education charity, organised this selection. All children available for child sponsorship from SOS Children are looked after in a family home by the charity. Read more...
| Antimony | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
51Sb
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Appearance | |||||||||||||||||||||||||
silvery lustrous gray |
|||||||||||||||||||||||||
| General properties | |||||||||||||||||||||||||
| Name, symbol, number | antimony, Sb, 51 | ||||||||||||||||||||||||
| Pronunciation | UK / ˈ æ n t ɨ m ə n i / AN-ti-mə-nee; US / ˈ æ n t ɨ m oʊ n i / AN-ti-moh-nee |
||||||||||||||||||||||||
| Metallic category | metalloid | ||||||||||||||||||||||||
| Group, period, block | 15 (pnictogens), 5, p | ||||||||||||||||||||||||
| Standard atomic weight | 121.760(1) | ||||||||||||||||||||||||
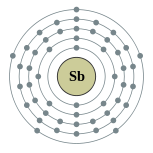
| Electron configuration | [Kr] 4d10 5s2 5p3 2, 8, 18, 18, 5 |
||||||||||||||||||||||||
| History | |||||||||||||||||||||||||
| Discovery | 3000 BC | ||||||||||||||||||||||||
| First isolation | Vannoccio Biringuccio (1540) | ||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||
| Density (near r.t.) | 6.697 g·cm−3 | ||||||||||||||||||||||||
| Liquid density at m.p. | 6.53 g·cm−3 | ||||||||||||||||||||||||
| Melting point | 903.78 K, 630.63 °C, 1167.13 °F | ||||||||||||||||||||||||
| Boiling point | 1860 K, 1587 °C, 2889 °F | ||||||||||||||||||||||||
| Heat of fusion | 19.79 kJ·mol−1 | ||||||||||||||||||||||||
| Heat of vaporization | 193.43 kJ·mol−1 | ||||||||||||||||||||||||
| Molar heat capacity | 25.23 J·mol−1·K−1 | ||||||||||||||||||||||||
| Vapor pressure | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||
| Oxidation states | 5, 3, -3 | ||||||||||||||||||||||||
| Electronegativity | 2.05 (Pauling scale) | ||||||||||||||||||||||||
| Ionization energies ( more) |
1st: 834 kJ·mol−1 | ||||||||||||||||||||||||
| 2nd: 1594.9 kJ·mol−1 | |||||||||||||||||||||||||
| 3rd: 2440 kJ·mol−1 | |||||||||||||||||||||||||
| Atomic radius | 140 pm | ||||||||||||||||||||||||
| Covalent radius | 139±5 pm | ||||||||||||||||||||||||
| Van der Waals radius | 206 pm | ||||||||||||||||||||||||
| Miscellanea | |||||||||||||||||||||||||
| Crystal structure | simple trigonal | ||||||||||||||||||||||||
| Magnetic ordering | diamagnetic | ||||||||||||||||||||||||
| Electrical resistivity | (20 °C) 417 nΩ·m | ||||||||||||||||||||||||
| Thermal conductivity | 24.4 W·m−1·K−1 | ||||||||||||||||||||||||
| Thermal expansion | (25 °C) 11 µm·m−1·K−1 | ||||||||||||||||||||||||
| Speed of sound (thin rod) | (20 °C) 3420 m·s−1 | ||||||||||||||||||||||||
| Young's modulus | 55 GPa | ||||||||||||||||||||||||
| Shear modulus | 20 GPa | ||||||||||||||||||||||||
| Bulk modulus | 42 GPa | ||||||||||||||||||||||||
| Mohs hardness | 3.0 | ||||||||||||||||||||||||
| Brinell hardness | 294 MPa | ||||||||||||||||||||||||
| CAS registry number | 7440-36-0 | ||||||||||||||||||||||||
| Most stable isotopes | |||||||||||||||||||||||||
| Main article: Isotopes of antimony | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
Antimony (Latin: stibium) is a chemical element with symbol Sb and atomic number 51. A lustrous gray metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient times and were used for cosmetics; metallic antimony was also known, but it was erroneously identified as lead. It was established to be an element around the 17th century.
For some time, China has been the largest producer of antimony and its compounds, with most production coming from the Xikuangshan Mine in Hunan. The industrial methods to produce antimony are roasting and subsequent carbothermal reduction or direct reduction of stibnite with iron.
The largest applications for metallic antimony are as alloying material for lead and tin and for lead antimony plates in lead-acid batteries. Alloying lead and tin with antimony improves the properties of the alloys which are used in solders, bullets and plain bearings. Antimony compounds are prominent additives for chlorine- and bromine-containing fire retardants found in many commercial and domestic products. An emerging application is the use of antimony in microelectronics.
Characteristics
Properties

Antimony is in the nitrogen group (group 15) and has an electronegativity of 2.05. As expected by periodic trends, it is more electronegative than tin or bismuth, and less electronegative than tellurium or arsenic. Antimony is stable in air at room temperature, but reacts with oxygen if heated to form antimony trioxide, Sb2O3.
Antimony is a silvery, lustrous gray metal that has a Mohs scale hardness of 3. Therefore, pure antimony is not used to make hard objects: coins made of antimony were issued in China's Guizhou province in 1931, but because of their rapid wear, their minting was discontinued. Antimony is resistant to attack by acids.
Four allotropes of antimony are known: a stable metallic form and three metastable forms (explosive, black and yellow). Metallic antimony is a brittle, silver-white shiny metal. When molten antimony is slowly cooled, metallic antimony crystallizes in a trigonal cell, isomorphic with that of the gray allotrope of arsenic. A rare explosive form of antimony can be formed from the electrolysis of antimony trichloride. When scratched with a sharp implement, an exothermic reaction occurs and white fumes are given off as metallic antimony is formed; when rubbed with a pestle in a mortar, a strong detonation occurs. Black antimony is formed upon rapid cooling of vapor derived from metallic antimony. It has the same crystal structure as red phosphorus and black arsenic, it oxidizes in air and may ignite spontaneously. At 100 °C, it gradually transforms into the stable form. The yellow allotrope of antimony is the most unstable. It has only been generated by oxidation of stibine (SbH3) at −90 °C. Above this temperature and in ambient light, this metastable allotrope transforms into the more stable black allotrope.
Metallic antimony adopts a layered structure ( space group R3m No. 166) in which layers consist of fused ruffled six-membered rings. The nearest and next-nearest neighbors form a distorted octahedral complex, with the three atoms in the same double-layer being slightly closer than the three atoms in the next. This relatively close packing leads to a high density of 6.697 g/cm3, but the weak bonding between the layers leads to the low hardness and brittleness of antimony.
Isotopes
Antimony exists as two stable isotopes, 121Sb with a natural abundance of 57.36% and 123Sb with a natural abundance of 42.64%. It also has 35 radioisotopes, of which the longest-lived is 125Sb with a half-life of 2.75 years. In addition, 29 metastable states have been characterized. The most stable of these is 124Sb with a half-life of 60.20 days, which has an application in some neutron sources. Isotopes that are lighter than the stable 123Sb tend to decay by β+ decay, and those that are heavier tend to decay by β- decay, with some exceptions.
Occurrence
The abundance of antimony in the Earth's crust is estimated at 0.2 to 0.5 parts per million, comparable to thallium at 0.5 parts per million and silver at 0.07 ppm. Even though this element is not abundant, it is found in over 100 mineral species. Antimony is sometimes found natively, but more frequently it is found in the sulfide stibnite (Sb2S3) which is the predominant ore mineral.
Compounds
Antimony compounds are often classified into those of Sb(III) and Sb(V). Relative to its congener arsenic, the +5 oxidation state is more stable.
Oxides and hydroxides
Antimony trioxide (Sb4O6) is formed when antimony is burnt in air. In the gas phase, this compound exists as Sb4O6, but it polymerizes upon condensing. Antimony pentoxide (Sb4O10) can only be formed by oxidation by concentrated nitric acid. Antimony also forms a mixed-valence oxide, antimony tetroxide (Sb2O4), which features both Sb(III) and Sb(V). Unlike phosphorus and arsenic, these various oxides are amphoteric, do not form well-defined oxoacids and react with acids to form antimony salts.
Antimonous acid Sb(OH)3 is unknown, but the conjugate base sodium antimonite ([Na3SbO3]4) forms upon fusing sodium oxide and Sb4O6. Transition metal antimonites are also known. Antimonic acid exists only as the hydrate HSb(OH)6, forming salts containing the antimonate anion Sb(OH)−
6. Dehydrating metal salts containing this anion yields mixed oxides.
Many antimony ores are sulfides, including stibnite (Sb2S3), pyrargyrite (Ag3SbS3), zinkenite, jamesonite, and boulangerite. Antimony pentasulfide is non-stoichiometric and features antimony in the +3 oxidation state and S-S bonds. Several thioantimonides are known, such as [Sb6S10]2− and [Sb8S13]2−.
Halides
Antimony forms two series of halides, SbX3 and SbX5. The trihalides SbF3, SbCl3, SbBr3, and SbI3 are all molecular compounds having trigonal pyramidal molecular geometry. The trifluoride SbF3 is prepared by the reaction of Sb2O3 with HF:
- Sb2O3 + 6 HF → 2 SbF3 + 3 H2O
It is Lewis acidic and readily accepts fluoride ions to form the complex anions SbF−
4 and SbF2−
5. Molten SbF3 is a weak electrical conductor. The trichloride SbCl3 is prepared by dissolving Sb2S3 in hydrochloric acid:
- Sb2S3 + 6 HCl → 2 SbCl3 + 3 H2S
The pentahalides SbF5 and SbCl5 have trigonal bipyramidal molecular geometry in the gas phase, but in the liquid phase, SbF5 is polymeric, whereas SbCl5 is monomeric. SbF5 is a powerful Lewis acid used to make the superacid fluoroantimonic acid ("HSbF6").
Oxyhalides are more common for antimony than arsenic and phosphorus. Antimony trioxide dissolves in concentrated acid to form oxoantimonyl compounds such as SbOCl and (SbO)2SO4.
Antimonides, hydrides, and organoantimony compounds
Compounds in this class generally are described as derivatives of Sb3-. Antimony forms antimonides with metals, such as indium antimonide (InSb) and silver antimonide (Ag3Sb). The alkali metal and zinc antimonides, such as Na3Sb and Zn3Sb2, are more reactive. Treating these antimonides with acid produces the unstable gas stibine, SbH3:
- Sb3− + 3 H+ → SbH3
Stibine can also be produced by treating Sb3+ salts with hydride reagents such as sodium borohydride. Stibine decomposes spontaneously at room temperature. Because stibine has a positive heat of formation, it is thermodynamically unstable and thus antimony does not react with hydrogen directly.
Organoantimony compounds are typically prepared by alkylation of antimony halides with Grignard reagents. A large variety of compounds are known with both Sb(III) and Sb(V) centers, including mixed chloro-organic derivatives, anions, and cations. Examples include Sb(C6H5)3 ( triphenylstibine), Sb2(C6H5)4 (with an Sb-Sb bond), and cyclic [Sb(C6H5)]n. Pentacoordinated organoantimony compounds are common, examples being Sb(C6H5)5 and several related halides.
History
Antimony(III) sulfide, Sb2S3, was recognized in predynastic Egypt as an eye cosmetic ( kohl) as early as about 3100 BC, when the cosmetic palette was invented.
An artifact, said to be part of a vase, made of antimony dating to about 3000 BC was found at Telloh, Chaldea (part of present-day Iraq), and a copper object plated with antimony dating between 2500 BC and 2200 BC has been found in Egypt. Austen, at a lecture by Herbert Gladstone in 1892 commented that "we only know of antimony at the present day as a highly brittle and crystalline metal, which could hardly be fashioned into a useful vase, and therefore this remarkable 'find' (artifact mentioned above) must represent the lost art of rendering antimony malleable."
Moorey was unconvinced the artifact was indeed a vase, mentioning that Selimkhanov, after his analysis of the Tello object (published in 1975), "attempted to relate the metal to Transcaucasian natural antimony" (i.e. native metal) and that "the antimony objects from Transcaucasia are all small personal ornaments." This weakens the evidence for a lost art "of rendering antimony malleable."
The first European description of a procedure for isolating antimony is in the book De la pirotechnia of 1540 by Vannoccio Biringuccio; this predates the more famous 1556 book by Agricola, De re metallica. In this context Agricola has been often incorrectly credited with the discovery of metallic antimony. The book Currus Triumphalis Antimonii (The Triumphal Chariot of Antimony), describing the preparation of metallic antimony, was published in Germany in 1604. It was purported to have been written by a Benedictine monk, writing under the name Basilius Valentinus, in the 15th century; if it were authentic, which it is not, it would predate Biringuccio.
Pure antimony was well known to Jābir ibn Hayyān in the 8th century. There is an ongoing controversy, with translator Marcellin Berthelot stating antimony was never found in Jābir's books, but others claiming that Berthelot translated only some of the less important books, while the more interesting ones (some of which might describe antimony) are not yet translated, and their content is completely unknown.
The first natural occurrence of pure antimony in the Earth's crust was described by the Swedish scientist and local mine district engineer Anton von Swab in 1783; the type-sample was collected from the Sala Silver Mine in the Bergslagen mining district of Sala, Västmanland, Sweden.
Etymology
The ancient words for antimony mostly have, as their chief meaning, kohl, the sulfide of antimony. Pliny the Elder, however, distinguishes between male and female forms of antimony; the male form is probably the sulfide, while the female form, which is superior, heavier, and less friable, has been suspected to be native metallic antimony.
The Egyptians called antimony mśdmt; in hieroglyphs, the vowels are uncertain, but there is an Arabic tradition that the word is ميسديميت mesdemet. The Greek word, στίμμι stimmi, is probably a loan word from Arabic or Egyptian sdm
|
and is used by Attic tragic poets of the 5th century BC; later Greeks also used στἰβι stibi, as did Celsus and Pliny, writing in Latin, in the first century AD. Pliny also gives the names stimi [ sic], larbaris, alabaster, and the "very common" platyophthalmos, "wide-eye" (from the effect of the cosmetic). Later Latin authors adapted the word to Latin as stibium. The Arabic word for the substance, as opposed to the cosmetic, can appear as إثمد ithmid, athmoud, othmod, or uthmod. Littré suggests the first form, which is the earliest, derives from stimmida, an accusative for stimmi.
The use of Sb as the standard chemical symbol for antimony is due to Jöns Jakob Berzelius, who used this abbreviation of the name stibium. The medieval Latin form, from which the modern languages and late Byzantine Greek take their names for antimony, is antimonium. The origin of this is uncertain; all suggestions have some difficulty either of form or interpretation. The popular etymology, from ἀντίμοναχός anti-monachos or French antimoine, still has adherents; this would mean "monk-killer", and is explained by many early alchemists being monks, and antimony being poisonous.
Another popular etymology is the hypothetical Greek word ἀντίμόνος antimonos, "against aloneness", explained as "not found as metal", or "not found unalloyed". Lippmann conjectured a hypothetical Greek word ανθήμόνιον anthemonion, which would mean "floret", and cites several examples of related Greek words (but not that one) which describe chemical or biological efflorescence.
The early uses of antimonium include the translations, in 1050–1100, by Constantine the African of Arabic medical treatises. Several authorities believe antimonium is a scribal corruption of some Arabic form; Meyerhof derives it from ithmid; other possibilities include athimar, the Arabic name of the metalloid, and a hypothetical as-stimmi, derived from or parallel to the Greek.
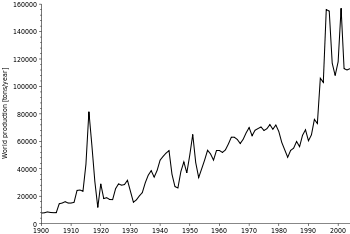
Production
Top producers and production volumes
The British Geological Survey reported that in 2005, the People's Republic of China was the top producer of antimony with an approximately 84% world share, followed at a distance by South Africa, Bolivia and Tajikistan. Xikuangshan Mine in Hunan province has the largest deposits in China with an estimated deposit of 2.1 million metric tons.
In 2010, according to the US Geological Survey, China accounted for 88.9% of total antimony production with South Africa, Bolivia and Russia sharing the second place.
| Country | Tonnes | % of total |
|---|---|---|
| 120,000 | 88.9 | |
| 3,000 | 2.2 | |
| 3,000 | 2.2 | |
| 3,000 | 2.2 | |
| 2,000 | 1.5 | |
| Top 5 | 131,000 | 97.0 |
| Total world | 135,000 | 100.0 |
However, Roskill Consulting estimates for primary production show that in 2010 China held a 76.75% share of world's supply with 120,462 tonnes (90,000 tonnes of reported and 30,464 tonnes of un-reported production), followed by Russia (4.14% share, 6,500 tonnes of production), Myanmar (3.76% share, 5,897 tonnes), Canada (3.61% share, 5,660 tonnes), Tajikistan (3.42% share, 5,370 tonnes) and Bolivia (3.17% share, 4,980 tonnes).
Roskill estimates that secondary production globally in 2010 was 39,540 tonnes.
Antimony was ranked first in a Risk List published by the British Geological Survey in the second half 2011. The list provides an indication of the relative risk to the supply of chemical elements or element groups required to maintain the current British economy and lifestyle.
Also, antimony was identified as one of 12 critical raw materials for the EU in a report published in 2011, primarily due to the lack of supply outside China.
Reported production of antimony in China fell in 2010 and is unlikely to increase in the coming years, according to the Roskill report. No significant antimony deposits in China have been developed for about ten years, and the remaining economic reserves are being rapidly depleted.
The world's largest antimony producers, according to Roskill, are listed below:
| Country | Company | Capacity (tonnes per year) |
|---|---|---|
| Mandalay Resources | 2,750 | |
| various | 5,460 | |
| Beaver Brook | 6,000 | |
| Hsikwangshan Twinkling Star | 55,000 | |
| Hunan Chenzhou Mining | 20,000 | |
| China Tin Group | 20,000 | |
| Shenyang Huacheng Antimony | 15,000 | |
| Kazzinc | 1,000 | |
| Kadamdzhai | 500 | |
| SRS | 500 | |
| US Antimony | 70 | |
| various | 6,000 | |
| GeoProMining | 6,500 | |
| Consolidated Murchison | 6,000 | |
| Unzob | 5,500 | |
| unknown | 600 | |
| Cengiz & Özdemir Antimuan Madenleri | 2,400 |
Reserves
According to statistics from the US Geological Survey (USGS), current global reserves of antimony will be depleted in 13 years. However, the United States Geological Survey expects more resources will be found.
| Country | Reserves (tonnes of antimony content) |
% of total |
|---|---|---|
| 950,000 | 51.88 | |
| 350,000 | 19.12 | |
| 310,000 | 16.93 | |
| 50,000 | 2.73 | |
| 21,000 | 1.15 | |
| Other countries | 150,000 | 8.19 |
| Total world | 1,831,000 | 100.0 |
Production process
The extraction of antimony from ores depends on the quality of the ore and composition of the ore. Most antimony is mined as the sulfide; lower grade ores are concentrated by froth flotation, while higher grade ores are heated to 500–600 °C, the temperature at which stibnite melts and is separated from the gangue minerals. Antimony can be isolated from the crude antimony sulfide by a reduction with scrap iron:
- Sb2S3 + 3 Fe → 2 Sb + 3 FeS
The sulfide is converted to an oxide and advantage is often taken of the volatility of antimony(III) oxide, which is recovered from roasting. This material is often used directly for the main applications, impurities being arsenic and sulfide. Isolating antimony from its oxide is performed by a carbothermal reduction:
- 2 Sb2O3 + 3 C → 4 Sb + 3 CO2
The lower grade ores are reduced in blast furnaces while the higher grade ores are reduced in reverberatory furnaces.
Applications
About 60% of antimony is consumed in flame retardants, and 20% is used in alloys for batteries, plain bearings and solders.
Flame retardants
Antimony is mainly used as its trioxide in making flame-proofing compounds. It is nearly always used in combination with halogenated flame retardants, with the only exception being in halogen-containing polymers. The formation of halogenated antimony compounds is the cause for the flame retarding effect of antimony trioxide, due to reaction of these compounds with hydrogen atoms and probably also with oxygen atoms and OH radicals, thus inhibiting fire. Markets for these flame-retardant applications include children's clothing, toys, aircraft and automobile seat covers. It is also used in the fibreglass composites industry as an additive to polyester resins for such items as light aircraft engine covers. The resin will burn while a flame is held to it but will extinguish itself as soon as the flame is removed.
Alloys
Antimony forms a highly useful alloy with lead, increasing its hardness and mechanical strength. For most applications involving lead, varying amounts of antimony are used as alloying metal. In lead–acid batteries, this addition improves the charging characteristics and reduces generation of unwanted hydrogen during charging. It is used in antifriction alloys (such as Babbitt metal), in bullets and lead shot, cable sheathing, type metal (for example, for linotype printing machines), solder (some " lead-free" solders contain 5% Sb), in pewter, and in hardening alloys with low tin content in the manufacturing of organ pipes.
Other applications
Three other applications make up nearly all the rest of the consumption. One of these uses is as a stabilizer and a catalyst for the production of polyethyleneterephthalate. Another application is to serve as a fining agent to remove microscopic bubbles in glass, mostly for TV screens; this is achieved by the interaction of antimony ions with oxygen, interfering the latter from forming bubbles. The third major application is the use as pigment.
Antimony is being increasingly used in the semiconductor industry as a dopant for heavily doped n-type silicon wafers in the production of diodes, infrared detectors, and Hall-effect devices. In the 1950s, tiny beads of a lead-antimony alloy were used to dope the emitters and collectors of n-p-n alloy junction transistors with antimony. Indium antimonide is used as a material for mid- infrared detectors.
Few biological or medical applications exist for antimony. Treatments principally containing antimony are known as antimonials and are used as emetics. Antimony compounds are used as antiprotozoan drugs. Potassium antimonyl tartrate, or tartar emetic, was once used as an anti- schistosomal drug from 1919 on. It was subsequently replaced by praziquantel. Antimony and its compounds are used in several veterinary preparations like anthiomaline or lithium antimony thiomalate, which is used as a skin conditioner in ruminants. Antimony has a nourishing or conditioning effect on keratinized tissues, at least in animals.
Antimony-based drugs, such as meglumine antimoniate, are also considered the drugs of choice for treatment of leishmaniasis in domestic animals. Unfortunately, as well as having low therapeutic indices, the drugs are poor at penetrating the bone marrow, where some of the Leishmania amastigotes reside, and so cure of the disease – especially the visceral form – is very difficult. Elemental antimony as an antimony pill was once used as a medicine. It could be reused by others after ingestion.
In the heads of some safety matches, antimony(III) sulfide is used. Antimony-124 is used together with beryllium in neutron sources; the gamma rays emitted by antimony-124 initiate the photodisintegration of beryllium. The emitted neutrons have an average energy of 24 keV. Antimony sulfides have been shown to help stabilize the friction coefficient in automotive brake pad materials.
Antimony also is used in the making of bullets and bullet tracers. This element is also used in traditional cosmetics, event paint and glass art crafts. An application as an opacifier in enamel declined in use after the 1930s, after several intoxications were reported.
Precautions
Antimony and many of its compounds are toxic, and the effects of antimony poisoning are similar to arsenic poisoning. The toxicity of antimony is by far lower than that of arsenic; this might be caused by the significant differences of uptake, metabolism and excretion between arsenic and antimony. The uptake of antimony(III) or antimony(V) in the gastrointestinal tract is at most 20%. Antimony(V) is not quantitatively reduced to antimony(III) in the cell (in fact antimony(III) is oxidised to antimony(V) instead).
Since methylation of antimony does not occur, the excretion of antimony(V) in urine is the main way of elimination. Like arsenic, the most serious effect of acute antimony poisoning is cardiotoxicity and the resulted myocarditis, however it can also manifest as Adams–Stokes syndrome which arsenic doesn't. Reported cases of intoxication by antimony equivalent to 90 mg antimony potassium tartrate dissolved from enamel has been reported to show only short term effects. An intoxication with 6 g of antimony potassium tartrate was reported to result in death after 3 days.
Inhalation of antimony dust is harmful and in certain cases may be fatal; in small doses, antimony causes headaches, dizziness, and depression. Larger doses such as prolonged skin contact may cause dermatitis, or damage the kidneys and the liver, causing violent and frequent vomiting, leading to death in a few days.
Antimony is incompatible with strong oxidizing agents, strong acids, halogen acids, chlorine, or fluorine. It should be kept away from heat.
Antimony leaches from polyethylene terephthalate (PET) bottles into liquids. While levels observed for bottled water are below drinking water guidelines, fruit juice concentrates (for which no guidelines are established) produced in the UK were found to contain up to 44.7 µg/L of antimony, well above the EU limits for tap water of 5 µg/L. The guidelines are:
- World Health Organization: 20 µg/L
- Japan: 15 µg/L
- United States Environmental Protection Agency, Health Canada and the Ontario Ministry of Environment: 6 µg/L
- German Federal Ministry of Environment: 5 µg/L