Cadmium
Did you know...
This selection is made for schools by a children's charity read more. See http://www.soschildren.org/sponsor-a-child to find out about child sponsorship.
| Cadmium | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
48Cd
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
silvery bluish-gray metallic |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name, symbol, number | cadmium, Cd, 48 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pronunciation | / ˈ k æ d m i ə m / KAD-mee-əm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metallic category | transition metal Alternatively considered a post-transition metal |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, period, block | 12, 5, d | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | 112.411 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Kr] 5s2 4d10 2, 8, 18, 18, 2 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Karl Samuel Leberecht Hermann and Friedrich Stromeyer (1817) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| First isolation | Karl Samuel Leberecht Hermann and Friedrich Stromeyer (1817) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Named by | Friedrich Stromeyer (1817) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 8.65 g·cm−3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Liquid density at m.p. | 7.996 g·cm−3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 594.22 K, 321.07 °C, 609.93 °F | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 1040 K, 767 °C, 1413 °F | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 6.21 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 99.87 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 26.020 J·mol−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vapor pressure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 2, 1 (mildly basic oxide) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.69 (Pauling scale) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies | 1st: 867.8 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd: 1631.4 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3rd: 3616 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | 151 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 144±9 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 158 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellanea | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | hexagonal close-packed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | (22 °C) 72.7 nΩ·m | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 96.6 W·m−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | (25 °C) 30.8 µm·m−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound (thin rod) | (20 °C) 2310 m·s−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 50 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 19 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 42 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.30 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 2.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 203 MPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 7440-43-9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Most stable isotopes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Main article: Isotopes of cadmium | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Like zinc, it prefers oxidation state +2 in most of its compounds and like mercury it shows a low melting point compared to transition metals. Cadmium and its congeners are not always considered transition metals, in that they do not have partly filled d or f electron shells in the elemental or common oxidation states. The average concentration of cadmium in the Earth's crust is between 0.1 and 0.5 parts per million (ppm). It was discovered in 1817 simultaneously by Stromeyer and Hermann, both in Germany, as an impurity in zinc carbonate.
Cadmium occurs as a minor component in most zinc ores and therefore is a byproduct of zinc production. It was used for a long time as a pigment and for corrosion resistant plating on steel while cadmium compounds were used to stabilize plastic. With the exception of its use in nickel–cadmium batteries and cadmium telluride solar panels, the use of cadmium is generally decreasing. These declines have been due to competing technologies, cadmium’s toxicity in certain forms and concentration and resulting regulations. Although cadmium has no known biological function in higher organisms, a cadmium-dependent carbonic anhydrase has been found in marine diatoms.
Characteristics
Physical properties
Cadmium is a soft, malleable, ductile, bluish-white divalent metal. It is similar in many respects to zinc but forms complex compounds. Unlike other metals, cadmium is resistant to corrosion and as a result it is used as a protective layer when deposited on other metals. As a bulk metal, cadmium is insoluble in water and is not flammable; however, in its powdered form it may burn and release toxic fumes.
Chemical properties
Although cadmium usually has an oxidation state of +2, it also exists in the +1 state. Cadmium and its congeners are not always considered transition metals, in that they do not have partly filled d or f electron shells in the elemental or common oxidation states. Cadmium burns in air to form brown amorphous cadmium oxide (CdO); the crystalline form of this compound is a dark red which changes colour when heated, similar to zinc oxide. Hydrochloric acid, sulfuric acid and nitric acid dissolve cadmium by forming cadmium chloride (CdCl2), cadmium sulfate (CdSO4), or cadmium nitrate (Cd(NO3)2). The oxidation state +1 can be reached by dissolving cadmium in a mixture of cadmium chloride and aluminium chloride, forming the Cd22+ cation, which is similar to the Hg22+ cation in mercury(I) chloride.
- Cd + CdCl2 + 2 AlCl3 → Cd2(AlCl4)2
Isotopes
Naturally occurring cadmium is composed of 8 isotopes. Two of them are naturally radioactive, and three are expected to decay but have not been experimentally confirmed to do so. The two natural radioactive isotopes are 113Cd ( beta decay, half-life is 7.7 × 1015 years) and 116Cd (two-neutrino double beta decay, half-life is 2.9 × 1019 years). The other three are 106Cd, 108Cd (both double electron capture), and 114Cd (double beta decay); only lower limits on their half-life times have been set. At least three isotopes – 110Cd, 111Cd, and 112Cd – are stable. Among the isotopes that do not occur naturally, the most long-lived are 109Cd with a half-life of 462.6 days, and 115Cd with a half-life of 53.46 hours. All of the remaining radioactive isotopes have half-lives that are less than 2.5 hours, and the majority of these have half-lives that are less than 5 minutes. Cadmium has 8 known meta states, with the most stable being 113mCd (t½ = 14.1 years), 115mCd (t½ = 44.6 days), and 117mCd (t½ = 3.36 hours).
The known isotopes of cadmium range in atomic mass from 94.950 u (95Cd) to 131.946 u (132Cd). For isotopes lighter than 112 u, the primary decay mode is electron capture and the dominant decay product is element 47 (silver). Heavier isotopes decay mostly through beta emission producing element 49 (indium).
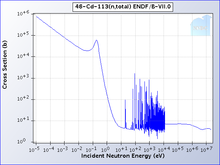
One isotope of cadmium, 113Cd, absorbs neutrons with very high probability if they have an energy below the cadmium cut-off and transmits them otherwise. The cadmium cut-off is about 0.5 eV. Neutrons with energy below the cut-off are deemed slow neutrons, distinguishing them from intermediate and fast neutrons.
Cadmium is created via the long s-process in low-medium mass stars with masses of 0.6 to 10 solar masses, which lasts thousands of years. It requires a silver atom to capture a neutron and then undergo beta decay.
History
Cadmium (Latin cadmia, Greek καδμεία meaning " calamine", a cadmium-bearing mixture of minerals, which was named after the Greek mythological character, Κάδμος Cadmus, the founder of Thebes) was discovered simultaneously in 1817 by Friedrich Stromeyer and Karl Samuel Leberecht Hermann, both in Germany, as an impurity in zinc carbonate. Stromeyer found the new element as an impurity in zinc carbonate (calamine), and, for 100 years, Germany remained the only important producer of the metal. The metal was named after the Latin word for calamine, since the metal was found in this zinc compound. Stromeyer noted that some impure samples of calamine changed colour when heated but pure calamine did not. He was persistent in studying these results and eventually isolated cadmium metal by roasting and reduction of the sulfide. The possibility to use cadmium yellow as pigment was recognized in the 1840s but the lack of cadmium limited this application.
Even though cadmium and its compounds may be toxic in certain forms and concentrations, the British Pharmaceutical Codex from 1907 states that cadmium iodide was used as a medication to treat "enlarged joints, scrofulous glands, and chilblains".
In 1927, the International Conference on Weights and Measures redefined the meter in terms of a red cadmium spectral line (1 m = 1,553,164.13 wavelengths). This definition has since been changed (see krypton).
After the industrial scale production of cadmium started in the 1930s and 1940s, the major application of cadmium was the coating of iron and steel to prevent corrosion; in 1944, 62% and in 1956, 59% of the cadmium in the United States was for coating. In 1956, 24% of the cadmium used within the United States was used for the second application, which was for red, orange and yellow pigments based on sulfides and selenides of cadmium. The stabilizing effect of cadmium-containing chemicals like the carboxylates cadmium laureate and cadmium stearate on PVC led to an increased use of those compounds in the 1970s and 1980s. The use of cadmium in applications such as pigments, coatings, stabilizers and alloys declined due to environmental and health regulations in the 1980s and 1990s; in 2006, only 7% of total cadmium consumption was used for plating and coating and only 10% was used for pigments. The decrease in consumption in other applications was made up by a growing demand of cadmium in nickel-cadmium batteries, which accounted for 81% of the cadmium consumption in the United States in 2006.
Occurrence
Cadmium makes up about 0.1 ppm of the Earth's crust. Compared with the more abundant 65 ppm zinc, cadmium is rare. No significant deposits of cadmium-containing ores are known. Greenockite (CdS), the only cadmium mineral of importance, is nearly always associated with sphalerite (ZnS). This association is caused by the geochemical similarity between zinc and cadmium which makes geological separation unlikely. As a consequence, cadmium is produced mainly as a byproduct from mining, smelting, and refining sulfidic ores of zinc, and, to a lesser degree, lead and copper. Small amounts of cadmium, about 10% of consumption, are produced from secondary sources, mainly from dust generated by recycling iron and steel scrap. Production in the United States began in 1907, but it was not until after World War I that cadmium came into wide use. One place where metallic cadmium can be found is the Vilyuy River basin in Siberia.
Rocks mined to produce phosphate fertilizers contain varying amounts of cadmium, leading to a cadmium concentration of up to 300 mg/kg in the produced phosphate fertilizers and thus in the high cadmium content in agricultural soils. Coal can contain significant amounts of cadmium, which ends up mostly in the flue dust.
Production
The British Geological Survey reports that in 2001, China was the top producer of cadmium, producing almost one-sixth of the world share, closely followed by South Korea and Japan.
Cadmium is a common impurity in zinc ores, and it is most often isolated during the production of zinc. Some zinc ores concentrates from sulfidic zinc ores contain up to 1.4% of cadmium. In 1970s, the output of cadmium was 6.5 pounds per ton of zinc. Zinc sulfide ores are roasted in the presence of oxygen, converting the zinc sulfide to the oxide. Zinc metal is produced either by smelting the oxide with carbon or by electrolysis in sulfuric acid. Cadmium is isolated from the zinc metal by vacuum distillation if the zinc is smelted, or cadmium sulfate is precipitated out of the electrolysis solution.
Applications
Cadmium has many common industrial uses as it is a key component in battery production, is present in cadmium pigments, coatings, and is commonly used in electroplating.
Batteries
In 2009, 86% of cadmium was used in batteries, predominantly in rechargeable nickel-cadmium batteries. Nickel-cadmium cells have a nominal cell potential of 1.2 V. The cell consists of a positive nickel hydroxide electrode and a negative cadmium electrode plate separated by an alkaline electrolyte ( potassium hydroxide). The European Union banned the use of cadmium in electronics in 2004 with several exceptions but reduced the allowed content of cadmium in electronics to 0.002%.
Electroplating
Cadmium electroplating, consuming 6% of the global production, can be found in the aircraft industry due to the ability to resist corrosion when applied to steel components. This coating is passivated by the usage of chromate salts. A limitation of cadmium plating is hydrogen embrittlement of high-strength steels caused by the electroplating process. Therefore, steel parts heat-treated to tensile strength above 1300 MPa (200 ksi) should be coated by an alternative method (such as special low-embrittlement cadmium electroplating processes or physical vapor deposition). In addition, titanium embrittlement caused by cadmium-plated tool residues resulted in banishment of these tools (along with routine tool testing programs to detect any cadmium contamination) from the A-12/SR-71 and U-2 programs, and subsequent aircraft programs using titanium.
Nuclear fission
Cadmium is used as a barrier to control neutrons in nuclear fission. The pressurized water reactor designed by Westinghouse Electric Company uses an alloy consisting of 80% silver, 15% indium, and 5% cadmium.
Compounds
Cadmium oxide is used in black and white television phosphors and in the blue and green phosphors for colour television picture tubes. Cadmium sulfide (CdS) is used as a photoconductive surface coating for photocopier drums.
In paint pigments, cadmium forms various salts, with CdS being the most common. This sulfide is used as a yellow pigment. Cadmium selenide can be used as red pigment, commonly called cadmium red. To painters who work with the pigment, cadmium yellows, oranges, and reds are the most brilliant and long-lasting colors to use. In fact, during production, these colors are significantly toned down before they are ground with oils and binders, or blended into watercolors, gouaches, acrylics, and other paint and pigment formulations. Since these pigments are potentially toxic, it is recommended to use a barrier cream on the hands to prevent absorption through the skin when working with them even though the amount of cadmium absorbed into the body through the skin is usually reported to be less than 1%.
In PVC, cadmium was used as heat, light, and weathering stabilizers. Currently, cadmium stabilizers have been completely replaced with barium-zinc, calcium-zinc and organo-tin stabilizers. Cadmium is used in many kinds of solder and bearing alloys, due to a low coefficient of friction and fatigue resistance. It is also found in some of the lowest-melting alloys, such as Wood's metal.
Laboratory uses
Helium–cadmium lasers are a common source of blue-ultraviolet laser light. They operate at either 325 or 422 nm and are used in fluorescence microscopes and various laboratory experiments. Cadmium selenide quantum dots emit bright luminescence under UV excitation (He-Cd laser, for example). The colour of this luminescence can be green, yellow or red depending on the particle size. Colloidal solutions of those particles are used for imaging of biological tissues and solutions with a fluorescence microscope.
Cadmium is a component of some compound semiconductors, such as cadmium sulfide, cadmium selenide, and cadmium telluride, which can be used for light detection or solar cells. HgCdTe is sensitive to infrared light and therefore may be utilized as an infrared detector or switch for example in remote control devices.
In molecular biology, cadmium is used to block voltage-dependent calcium channels from fluxing calcium ions, as well as in hypoxia research to stimulate proteasome-dependent degradation of Hif-1α.
Biological role
Cadmium has no known useful role in higher organisms, but a cadmium-dependent carbonic anhydrase has been found in some marine diatoms. The diatoms live in environments with very low zinc concentrations and cadmium performs the function normally carried out by zinc in other anhydrases. The discovery was made using X-ray absorption fluorescence spectroscopy (XAFS).
The highest concentration of cadmium has been found to be absorbed in the kidneys of humans, and up to about 30 mg of cadmium is commonly inhaled throughout childhood and adolescence.
Cadmium can be used to block calcium channels in chicken neurons.
Safety
The most dangerous form of occupational exposure to cadmium is inhalation of fine dust and fumes, or ingestion of highly soluble cadmium compounds. Inhalation of cadmium-containing fumes can result initially in metal fume fever but may progress to chemical pneumonitis, pulmonary edema, and death.
Cadmium is also an environmental hazard. Human exposures to environmental cadmium are primarily the result of fossil fuel combustion, phosphate fertilizers, natural sources, iron and steel production, cement production and related activities, nonferrous metals production, and municipal solid waste incineration. Bread, root crops, and vegetables also contribute to the cadmium in modern populations. There have been a few instances of general population toxicity as the result of long-term exposure to cadmium in contaminated food and water, and research is ongoing regarding the estrogen mimicry that may induce breast cancer. In the decades leading up to World War II, Japanese mining operations contaminated the Jinzū River with cadmium and traces of other toxic metals. As a consequence, cadmium accumulated in the rice crops growing along the riverbanks downstream of the mines. Some members of the local agricultural communities consuming the contaminated rice developed itai-itai disease and renal abnormalities, including proteinuria and glucosuria.
The victims of this poisoning were almost exclusively post-menopausal women with low iron and other mineral body stores. Similar general population cadmium exposures in other parts of the world have not resulted in the same health problems because the populations maintained sufficient iron and other mineral levels. Thus, while cadmium is a major factor in the itai-itai disease in Japan, most researchers have concluded that it was one of several factors. Cadmium is one of six substances banned by the European Union's Restriction on Hazardous Substances (RoHS) directive, which bans certain hazardous substances in electrical and electronic equipment but allows for certain exemptions and exclusions from the scope of the law.
Although some studies linked exposure to cadmium with lung and prostate cancer, there is still a substantial controversy about the carcinogenicity of cadmium. More recent studies suggest that arsenic rather than cadmium may lead to the increased lung cancer mortality rates. Furthermore, most data regarding the carcinogenicity of cadmium rely on research confounded by the presence of other carcinogenic substances.
Tobacco smoking is the most important single source of cadmium exposure in the general population. It has been estimated that about 10% of the cadmium content of a cigarette is inhaled through smoking. The absorption of cadmium from the lungs is much more effective than that from the gut, and as much as 50% of the cadmium inhaled via cigarette smoke may be absorbed.
On average, smokers have 4–5 times higher blood cadmium concentrations and 2–3 times higher kidney cadmium concentrations than non-smokers. Despite the high cadmium content in cigarette smoke, there seems to be little exposure to cadmium from passive smoking. No significant effect on blood cadmium concentrations has been detected in children exposed to environmental tobacco smoke.
Cadmium exposure is a risk factor associated with early atherosclerosis and hypertension, which can both lead to cardiovascular disease.
Regulations
Due to the adverse effects on the environment and human health, the supply and use of cadmium is restricted in Europe under the REACH Regulation.
Product recalls
In May 2006, a sale of the seats from Arsenal F.C.'s old stadium, Highbury in London, England was cancelled after the seats were discovered to contain trace amounts of cadmium. Reports of high levels of cadmium use in children's jewelry in 2010 led to a US Consumer Product Safety Commission investigation. The U.S. CPSC issued specific recall notices for cadmium content applying to jewelry sold by Claire's and Wal-Mart stores. In June 2010 McDonald's voluntarily recalled more than 12 million promotional "Shrek Forever After 3D" Collectable Drinking Glasses owing to concerns over cadmium levels in paint pigments used on the glassware. The glasses were manufactured by Arc International, of Millville, NJ, USA.